- By Profab /
- August 8, 2025
Surface treatment is the process of changing the surface properties of an object by physical, chemical or electrochemical means. Its core purpose is to enhance specific properties of a material, such as wear resistance, corrosion resistance, lubricity, reflectivity, electrical conductivity, or overall appearance.
Surface treatments are significant in enhancing material performance, durability and aesthetics. They are critical to extending product life and reducing maintenance requirements, as they effectively prevent rust and degradation. Surface treatments are especially essential for components exposed to harsh environments such as moisture, aggressive chemicals, or biological fluids.
In this article, we will take an in-depth look at the various types of surface treatment technologies. The two main categories are surface coating technology and surface conversion film technology. Start exploring with Profab Machine!
Table of Contents
Surface Coating Technologies
(Electroplating, Electrophoretic Coating, Spray Painting, Powder Coating, etc.)
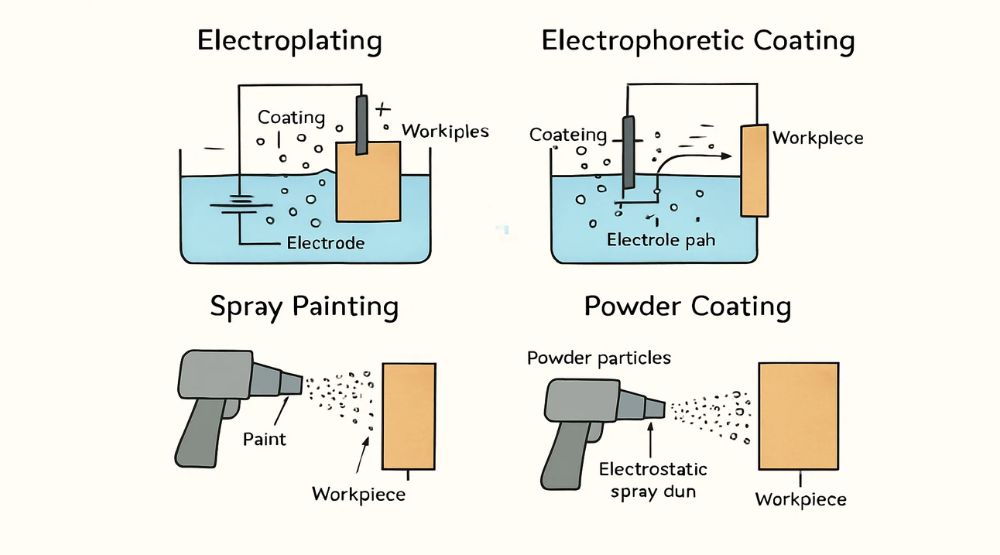
Electroplating
Electroplating is an electrochemical deposition method that forms a dense, uniform, and highly adherent metal or alloy coating on a substrate surface through controlled current flow. Common electroplating types include zinc plating, chrome plating, and nickel plating.
Basic Electroplating Workflow
Pre-treatment: Degreasing, acid pickling, rinsing
Electroplating Stage: Anode, cathode, electrolyte bath
Post-treatment: Sealing, passivation, hydrogen removal
Precise control of current density, electrolyte composition, and temperature is crucial to achieve a pure, uniform coating with excellent adhesion. For instance, pulse electroplating demands high-performance power supplies to manage complex current waveforms. Any deviation can lead to defects, highlighting that successful electroplating is a tightly regulated chemical-electrical process.
Advantages & Limitations of Electroplating
Advantages
Enhanced Surface Performance: Improves wear resistance, corrosion resistance, lubrication, reflectivity, conductivity, and aesthetic finish.
Thickness Build-Up: Restores worn parts or adds thickness to undersized components, extending service life.
Complex Geometries: Enables electroforming of intricate metal parts.
Specialized Applications: Deposits conductors in PCBs and ICs; refines metal purity; brush plating repairs bearing surfaces via nickel or silver deposition.
Limitations
Critical Cleanliness: Even microscopic oil films or contaminants can severely impair adhesion.
Process Control: Pulse plating requires sophisticated power supplies; reverse plating risks anode contamination.
Operator Skill-Dependence: Brush plating demands high operator expertise and may yield uneven thickness.
Surface Wear: Barrel plating is efficient for small parts but can cause surface abrasion.
Purpose: Increases surface hardness, decorative finish, rust prevention
Applications: Tie-rod balls, shaft bodies, ball heads
Thickness:
Decorative chrome: 0.5–1 μm
Hard chrome: 5–80 μm
Purpose: Wear resistance, corrosion protection, rust prevention
Applications: Tie-rod SA series
Thickness: 10–30 μm
Purpose: Decorative finish, rust prevention
Applications: Ball bearing sleeves, metal tubes, tie-rod bodies, threaded nuts, ball head housings
Thickness: 5–15 μm
Cost Ranking: Chrome > Nickel > Zinc
Electrophoretic Coating
Electrophoretic coating deposits pigment- and resin-laden liquids onto a workpiece via an electric field, creating a uniform film for protection, decoration, and enhanced surface performance. Most e-coats are black or gray; bright colors are less common due to heat-induced discoloration and higher costs.
Process Flow
Pre-treatment: Degreasing, rust removal
Anodic electrophoresis
Post-treatment: Rinse → Bake (film thickness: 15–25 μm)
Tips: For maximum surface hardness and wear resistance, choose electroplating; for superior corrosion resistance and finish quality, choose electrophoretic coating.
Powder Coating
Powder coating uses electrostatically charged plastic powder that adheres to the metal surface and then melts and cures under high-temperature baking to form a durable finish.
Typical Film Thickness: 60–70 μm
Curing Temperature: ~180 °C
Advantages & Limitations of Powder Coating
Advantages
Exceptional durability against abrasion, scratches, corrosion, and chemicals
Long-lasting, fade-resistant appearance; virtually unlimited color and texture options (gloss, matte, metallic, clear)
Thick coatings achievable in a single pass; high transfer efficiency (≥95 %)
Environmentally friendly: solvent-free, negligible VOCs, minimal regulatory burden
Cost-effective over the long term due to extended service life
Fast, automated processing with high throughput and minimal waste
Low operator training requirements; simple maintenance
Limitations
Film-thickness variations can cause optical defects; very thin films (<1 mil) prone to pinholes
Frequent color changes increase downtime
Requires climate-controlled storage
Color matching more challenging than liquid paint
Difficult to uniformly coat sharp edges and recesses; Faraday cage effect
High curing temperatures may not suit heat-sensitive parts
Transitioning from liquid to powder systems involves significant capital investment
Particle size consistency is critical; reclaimed powder demands strict quality control
Spray Painting
Spray painting applies liquid coatings via a spray gun, followed by baking at lower temperatures than powder coating.
Typical Film Thickness: Thinner than powder coating
Curing Temperature: ~80 °C
Comparison Of Painting, Plastic Spraying And Electrophoresis
| Technology Comparison | Film Thickness | Curing Temperature | Key Benefits |
| Spray Painting | Thinner (10–30 μm) | ~80 °C | Smooth finish, easy touch-up |
| Powder Coating | 60–70 μm | ~180 °C | High durability, no VOCs |
| Electrophoretic Coating | 15–25 μm | ~150–160 °C | Uniform coverage, corrosion resistance |
Surface Conversion Coatings
(Blackening/Bluing, Phosphating, Anodizing)
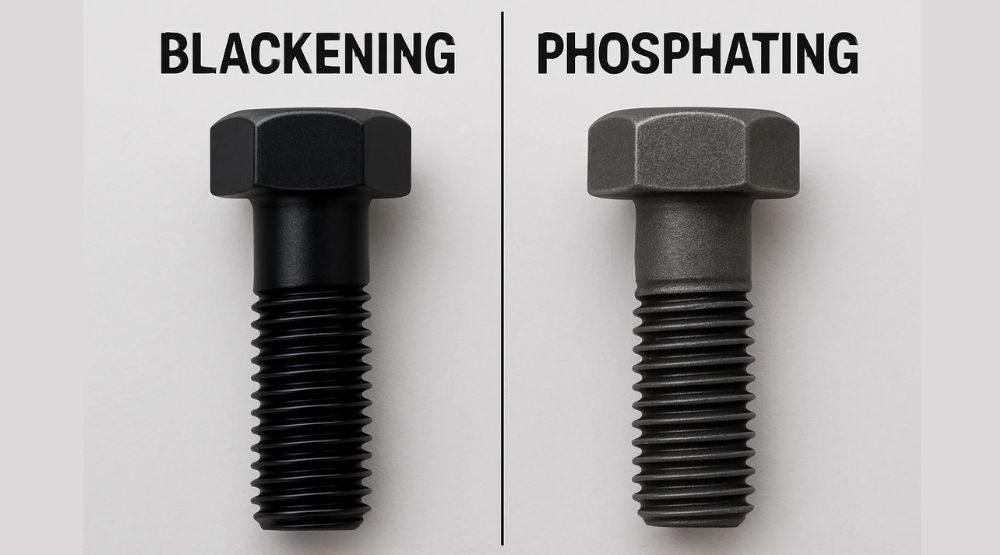
Blackening & Bluing
Blackening: A thermal process forming an oxide layer on steel to block air and prevent rust. Often called “bluing” when applied to steel.
Bluing: A chemical conversion that creates a dense oxide film for corrosion resistance and wear protection without affecting the base metal’s microstructure.
Phosphating (Chemical Conversion Coating)
Phosphating is a common pre-treatment where steel, aluminum, or zinc parts are immersed in a phosphate-based solution, depositing a water-insoluble crystalline film for enhanced corrosion protection.
Blackening vs. Phosphating
Corrosion resistance: Phosphating > Blackening
Appearance: Blackening yields a shinier black finish
Cost: Phosphating is more expensive. Both require thorough pre-cleaning (rust removal) to avoid early corrosion.
Anodizing (Electrochemical Oxide Conversion)
Anodizing generates a thin, dense oxide layer on non-ferrous metals (primarily aluminum and its alloys) by making the part the anode in an electrolytic bath.
Anodizing Steps
1)Cleaning: Alkali or acid wash to remove oils and impurities.
2)Pre-treatment: Deburring, sandblasting, grinding, or polishing.
3)Anodizing: Electrolytic oxidation (film thickness controlled by electrolyte, current, and time).
4)Coloring: Dye immersion.
5)Sealing: Hot water or sealant immersion to close pores, boosting corrosion resistance and hardness.
6)Final Rinse & Dry
Advantages & Limitations of Anodizing
Advantages
Excellent corrosion resistance against moisture, oxygen, UV, heat, and marine environments
Improved electrical insulation while maintaining substrate conductivity
Hard, wear-resistant finish that extends component life
Highly resistant to chipping or flaking
Limitations
Requires specialized equipment; higher cost
Color may fade under prolonged UV exposure
Susceptible to physical damage from sharp impacts
Certain alloy compositions (high copper or silicon) can impair coating quality
The above are common types of surface treatments used in industrial applications. Profab Machine’s stainless steel components also offer a wide range of surface treatment services. We look forward to your exploration.
FAQ
Is Anodizing UV-Resistant?
The oxide layer resists corrosion but can degrade under intense UV exposure. Hard anodizing also lacks UV immunity.
Mitigation Strategies
Apply a UV-blocking topcoat (similar to spray paint)
Optimize electrolyte formulation and extend sealing time
Use UV-stable dyes
Hard Anodizing vs. Standard Anodizing
| Feature | Standard Anodizing | Hard Anodizing |
| Film Thickness | 5–12 μm | 25–70 μm |
| Hardness (HV) | 180–310 | 310–550 |
| Surface Finish | Smoother, transparent | Slightly rougher, opaque |
| Dimensional Change | Minimal | More pronounced |
Send Inquiry Now
Related Resource

Advantages of Maintenance-Free Rod Ends in Hydraulic Applications

Load Capacity Considerations For Clevis Rod Ends In Construction Machinery

Stainless Welded Tubing vs Seamless Tubing: The Complete Comparison Guide
